Abstract
|
The phenylalanine ammonia-lyase is a key enzyme in phenylpropanoid synthesis, a pathway
for the biosynthesis of a
widerange of natural products which play key roles in plant development and protection
agianst environmental stresses
including the structural polymer lignin, flavonoids (anthocyanin pigments and UV
protectants), isoflavonoids and phytoalexins
(Fig. 1). In this study, PSPAL2, a member of pea PAL gene family was
determined and structurally characterized. The structure
f PSPAL2 was divided into two exons by the single intron of 90 bp. The deduced
amino acid sequence of PSPAL2 showed
that the gene encode 724 amino acids with a deduced molecular weight of 79,005 Da.. In PSPAL2,
we have found the
retrotransposon-like sequence in the 5/-upstream region of PSPAL2 promoter
(Fig. 2A). Moreover, we have found some
putative cis-regulatory elements of box I, box II, and box IV which are conserved
among the promoter of several genes
involved in the phenylpropanoid pathway (Fig. 2B).
To discriminate
the function of PSPAL2 promoter, we have demonstrated the expression of pea PSPAL2
retrotransposon
-like sequence in the region between -406 and -2196 and the three types of sequentially
deleted chimeric promoter constructs
designated as PSPAL2-FLd1, PSPAL2-FLd2 and PSPAL2-FLd3 in transgenic tobacco
during developmental growth and upon
fungal ingression. The histochemical GUS expression in young seedlings and mature plants
seems to be conserved in tissue
and organ-specific expressions (roots, stems, leaves, flower organs and anthers).
Moreover, the levels of GUS activities in
tissues of transgenic plants depend on the 5/-upstream region of PSPAL2 promoter
were also determined. Extremely low
GUS expression was observed in healthy or undisturbed mature leaves. However, the
PSPAL2 promoter activated in leaves
of transgenic tobacco plants after transfer to the greenhouse was induced upon fungal
ingression, especially when leaves
inoculated with P. capsici were incubated at 22-24oC for 48 hr. Marked
expression was detected at the HR area surrounding
the inoculation site of the transformant of PSPAL2-FL. Extremely low GUS expression
was observed in the transformant of
PSPAL2-FLd3. Although brown necrotic regions were observed at the cross cut. The
results demonstrated that the region from
-966 to -2196 of PSPAL2 promoter played a crucial role in the regulation of
induction of GUS activities in the mature leaves of
transgenic tobacco plants. It is thus clear that the 5/-upstream region between
+110 to -594 is insufficient to establish the full
capacity of defense gene response under stress even though this region contains important
box sequences such as box I,
box II and box IV. The additional sequences from -594 to -2196 that include the region of
retrotransposon-like sequence are
obviously necessary for the functional expression of the gene not only during
developmental growth but also in response to
fungal ingress and injuries. |
Methods and
Materials |
1. Construction of chimeric genes
The pea PSPAL2 full length promoter (PSPAL2-FL,
-2196 to +110) and three deleted chimeric promoters designated as
PSPAL2-FLd1 (-1486 to +110), PSPAL2-FLd2 (-966 to +110)
and PSPAL2-FLd3 (-594 to +110) had been constructed into CAT
reporter gene for analyzing the transient expression in electroporated protoplasts (Yamada
et al., 1994). To investigate the
expression of PSPAL2 promoters in transgenic tobacco plants, the PSPAL2-FL
promoter and the three selected deletion
construct promoters in the CAT reporter gene were amplified by PCR using specific primers
and subcloned into pBluescriptII
KS (+) at Hind III and Bam HI sites, then ligated with the
GUS reporter gene in pBI101.2 (Fig. 3). The PSPAL2- GUS chimeric
promoter constructs were purified and transformed into Agrobacterium tumefaciens
LBA4404 by the freeze-thaw method
(Holster et al., 1978)
2. Preparation of sterile tobacco plants
Nicotiana tabacum was used in the transformation
experiments. Seeds were surface-sterilized in 5% hypochlorite for 10
min, followed by soaking in 70% ethanol for 10 min, and rinsed in sterilized water.
Sterilized seeds were germinated in a sterile
petri dish containing the MS medium (Murashige and Skoog, 1962)
3. Leaf disk transformation
Leaf disk of the sterilized tobacco was co-cultured in MS
medium for 15-30 min with 1-5 x 108 cell/ml of A. tumefaciens
LBA4404 (McCormick et al., 1986) carrying the specified chimeric genes as will be
described (Fig. 4A). After drying on a
sterilized Whatman 3 MM filter paper to remove excess bacteria, inoculated leaves were
placed abaxial surface down on MS
medium containing 1.0 mg/l of NAA and 1.0 mg/l of BA. The inoculated leaves were incubated
at 22-24oC for 2 days, and then,
transferred onto MS-selective medium containing the same concentration of NAA, BA,
Kanamycin (100 mg/l) and claforan (500
mg/l) until shoots formed. After 2-3 weeks of incubation, adventitious shoots were
transferred to new MS medium until roots
formed (Gelvin et al., 1990). All plant materials were incubated at 25-280C
under a 16 -hr. light (150 m E / m2 /s), 8-hr dark
conditions. Young seedlings (Fig. 4B, 4C) were transplanted into soil and incubated in a
greenhouse (Fig. 4D).
4. GUS histochemical assay and PCR analysis
GUS histochemical assay
Mature leaves were fixed by soaking in 1% formaldehyde in
50 mM sodiumphosphate buffer (pH 7.0) for 10 min and rinsed
three times with 50 mM sodiumphosphate buffer (pH 7.0). Then, they were incubated in
X-Gluc solution (1.0 mM 5-bromo-4
-chloro-3-indolyl- b -D-glucuronide, in 50 mM. sodiumphosphate buffer (pH 7.0), 0.5 mM
potassium ferricyanide, 0.5 mM
potassium ferrocyanide, 10 mM EDTA) at 370C for overnight as described by
Jefferson et al. (1987). Straining and fixation of
X-Gluc solution into tissues was facilitated by vacuum infiltration. Staining reactions
were stopped by transferring the tissues
into 70% ethanol.
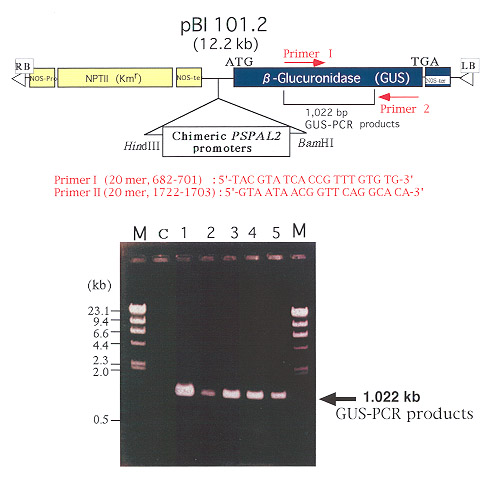
GUS-PCR analysis
Genomic DNA extraction for GUS-PCR was performed to
confirm the integration of PSPAL2-GUS fusions in the genome
of transgenic tobacco plants as described by Hosaka (1994). GUS-PCR was amplified using
GUS-specific primers [primer I
(upstream) 20 mer : 5/-TAC GTA TCA CCG TTT GTG TG-3/; primer II
(down stream) 20 mer : 5/-GTA ATA ACG GTT CAG
GCA CA -3/] (Fig. 5). DNA manipulation was performed according to the standard
methods described by Sambrook et al.,
(1989) or as specified by the manufacturer’s protocols. |
Results and Discussion
1. GUS
expression in transgenic tobacco plants during developmental growth
We have observed basal GUS expression of PSPAL2-FL
promoter in tissues of roots, stems and leaves during
developmental growth of young seedling before transplanting to soil. The histochemical GUS
expression in young
seedlingsand mature plants seems to be conserved in tissue and organ-specific expressions
as observed in PSPAL1
( Kawamata et al., 1997 ) and bean PAL2 (Shufflebottom et at., 1993 and
Hatton et al., 1995). PSPAL2 promoter showed
strong GUS expression in xylem, phloem elements of the vascular and endodermal tissues of
lateral roots (Fig. 6), stems
(Fig. 7) and vascular tissues in veins of leaves, leaf tips, and petrioles (Fig. 8).
Strong GUS activity was also found in flower
organs,especially in the pigment parts of petals, sepal tips, gland cells of trichomes
(Fig. 9) and anthers (Fig. 10). However,
we could not observe the GUS expression in root hairs as found in PSPAL1.
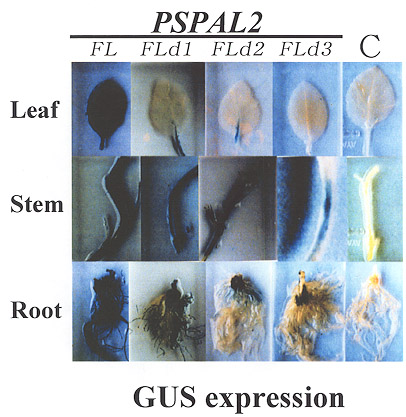
Moreover, the level of GUS activities in organ
of transgenic plants significantly declined in corresponding to the deleted 5/-upstream
chimeric PSPAL2 promoters from
-2196 to -594,especially in roots, vascular tissues in veins of leaves and stems as shown
in Fig. 11.
2. GUS expression in
transgenic tobacco plants upon fungal ingression and injuries
Histochemical GUS expression
The leaves of transgenic tobacco plants carrying PSPAL2-FL
were inoculated with Phytophthora nicotianae, a tobacco
pathogenic fungi (P) or with P. capsici, a nonpathogen (NP). The results showed
that histochemically detected GUS
expression in transgenic tobacco plants was the highest at 48 hr after inoculation with
P. capsici (Fig. 12B) and incubated at
22-240C. Then GUS expression gradually faded away at the hypersensitive
response (HR) area around the inoculation site
after 72 hr of incubation (Fig. 12C), where a plant defense system had presumably been
established for blocking fungal
invasion, in a manner similar to the expression of PSPAL1 promoter
(Kawamata et al., 1997). The pattern of the GUS
xpression after inoculation with a pathogen was not as clear as one observed in the
necrotic area after inoculation with a
nonpathogen and never faded until the whole leaf was colonized.
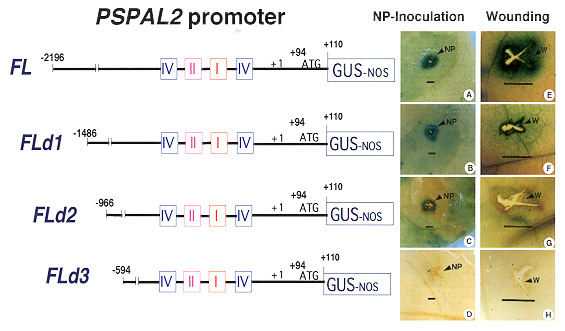
Expression of deleted PSPAL2 promoters upon fungal
infection
To discriminate the expression of the deleted PSPAL2
promoters upon fungal infection, mature leaves of transgenic
tobacco plants carrying the PSPAL2 chimeric promoter constructs were inoculated
with P. capsici. This nonpathogenic
fungus induced a very large, clear GUS expression zone around the site of the
hypersensitive response (HR), especially
in the transformants of PSPAL2-FL (Fig. 13A) and PSPAL-FLd1 (Fig. 13B) at 48
hr after the inoculation. However, the responses
to fungi infection were not high in the transformants of PSPAL-FLd2 (Fig. 13C); the
expression zone was restricted to the area
around the inoculation site. GUS expression in the transformant of PSPAL-FLd3 did
not clearly appear (Fig. 13D). The results
showed that the pea PSPAL2 promoter expression in transgenic tobacco was strongly
affected by the sequences in 5/-
upstream region as previously shown with the transient CAT expression in electroporated
pea protoplasts (Yamada et al.,
1994). The functional analysis of 5/-nested deletions of PSPAL2 promoter
in electroporated protoplasts showed that an
enhancer-like element is located at the TATA-distal region from -2196 to-406, and this
promoter was activated by fungal
elicitor from M. pinodes and partially suppressed by the suppressor from the same
fungus (Yamada et al., 1994). Interestingly,
the GUS expression of our constructs upon fungal ingression in transgenic tobacco leaves
demonstrated the induction of
positive defense responses at the sites of infection at different levels depending on the
additional sequences of the 5/-
upstream region. Because we have observed a very low level of the GUS expression in the
transformants of PSPAL2-FLd3
compared to PSPAL2-FL, the lower level of GUS expression is not due to a position
effect of the integration of the chimeric
promoters. This phenomenon may be explained by
the lack of some active elements that are needed for the regulation of the
PSPAL2 promoter. These elements are likely to span from -966 to -2196. Moreover, we
have observed GUS expression in
mature transgenic tobacco leaves injured with sterile razor blade histochemically. Intense
blue colorations were observed at
wounding sites (W) and restricted adjacent areas. The GUS expression of the deleted PSPAL2-FL
promoters after wounding
also declined from a high level to an extremely low one as the deletion was extended, in a
manner similar to the expression
after fungal ingression (Fig. 13E-H).
The results demonstrated that the region from -966 to -2196 of PSPAL2
promoter played a crucial role in the regulation of
induction of GUS activities in the mature leaves of transgenic tobacco plants. It is thus
clear that the 5/-upstream region
between +110 to -594 is insufficient to establish the full capacity of defense gene
response under stress even though this
region contains important box sequences such as box I, box II and box IV. The additional
sequences from -594 to -2196 that
include the region of retrotransposon-like sequence are obviously necessary for the
functional expression of the gene not
only during developmental } growth but also in response to fungal ingress and injuries. |
References
|
Gelvin, S.B., Schilperoort, R.A., and
Verma, D.P.S. 1990. Plant Molecular Biology Manual. Kluwer
Academic Publishers, NY.
Hatton, D., Sablowski, R., Yung, M.H., Smith, C., Schuch, W. and Bevan, M. 1995. Two
classes of cis
sequences contribute to
tissue-specific expression of a PAL2 promoter in transgenic tobacco.
Plant J. 7(6):859-876.
Holsters, M., de Waele, D., Depicker, A., Messens, E., van Montagu, N. and Schell, J.
1978.
Transfection and transformation of Agrobacterium
tumefaciens. Mol. Gen. Genet. 163(2):
181-187.
Hosaka, K. 1994. Current RAPD technology. Academic booklet, Kobe University, Kobe.
Jefferson, R A., Kavanagh, T.A. and Bevan, M.W. 1987. GUS fusion: b -glucuronidase as a
sensitive and versatile gene marker in
higher plants. EMBO J. 6: 3901-3907.
Kawamata, S., Shimoharai, K., Imura, Y., Ozaki, M., Ichinose, Y., Shiraishi, T., Kunoh, H
and
Yamada, T. 1997. Temporal and spatial
pattern of expression of the pea phenylalanine
ammonia-lyase gene1 promoter in
transgenic tobacco. Plant Cell Physiol. 38(7): 792-803.
McCormick, S., Niedermeyer, J. and Fry, J. 1986. Leaf disc transformation of cultivated
tomato
(L. esculentum) using Agrobacterium
tumefaciens. Plant Microbe Interact. 6(4): 453-466.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with
tobacco tissue cultures. Physiol.
Plant. 15: 473-497.
Sambrook, T., Fritsch, E.F. and Manitis, J. 1989. Molecular Cloning: A Laboratory Manual.
Cold
Spring Habor, NY.
Shufflebottom, D., Edwards, K., Schuch, W. and Bevan, M. 1993. Transcription of two
members
of a gene family encoding phenylalanine
ammonia-lyase leads to remarkably different cell
specificities and induction
patterns. Plant J. 3(6): 835-845.
Sriprasertsak, P. 2000. Plant defense responses and control of gene expression: Structure
and
function of the promoter of PSPAL2,
a pea defensive gene encoding phenylalanine
ammonia-lyase. Dissertation Ph.D, Okayama
University, Japan.
Yamada, T., Sriprasertsak, P., Kato, H., Hashimoto, T., Shimizu, H., and Shiraishi, T.
1994.
Functional analysis of the promoters of
phenylalanine ammonia-lyase genes in pea. Plant
Cell Physiol. 35: 917-926.
|

|
Fig. 1 The
phenylpropanoid biosynthesis pathway
PAL
: phenylalanine ammonia-lyase, C4H : cinnamic acid 4 - hydroxylase
4CL : 4 -
coumaric acid CoA ligase, CHS : chalcone synthase, CHI :
Chalcone isomerase
CCR : 4 - coumaroyl CoA
reductase, CAD : coumaroyl alcohol dehydrogenase |

|
Fig. 2A
The retrotransposon-like sequence in the 5/-upstream region of PSPAL2
promoter |
|
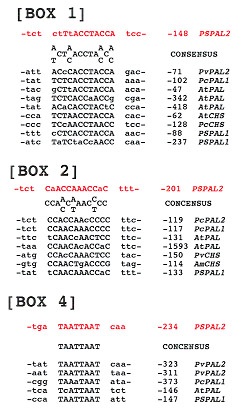
Fig. 2B
Conserved sequence motifs in the promoters of several phenylpropanoid biosynthesis genes
PAL : phenylalanine ammonia-lyase, CHS :
chalcone synthase
Pv : Phaseolus vulgaris, Pc : Petroselinum
crispum, At : Arabidopsis thaliana,
Am : Antirrhinum majus, Ps : Pisum
sativum
|
 |
Fig. 3
Schematic representation of PSPAL2
promoter-GUS-NOS fusion. Full length (PSPAL2-
FL) promoter-sequence and the three deleted
chimeric promoter constructs ( PSPAL-FLd1,
PSPAL-FLd2 and PSPAL-FLd3 ) were fused to
GUS in pBI101.2 (Toyobo Inc, Kyoto, Japan) at
HindIII and BamHI sites. The nucleotide
sequences of all chimeric promoter constructs,
the positions where GUS-NOS cassette were
connected, a putative translation initiation codon
and transcription start site (TXN) are indicated.
Putative TATA box, CAAT box and characteristic
sequences motifs such as box I, II and IV in 5/-
upstream region relative to the transcriptional
start site are denoted by colors with those for
PSPAL2 being beneath them. The numbers on
top denote the nucleotide position from the
transcriptional start point. GUS and NOS: b
-glucuronidase gene in pBI101.2 and
mkAgrobacterium tumefaciens nopaline synthase
gene terminator sequence. |
|
|
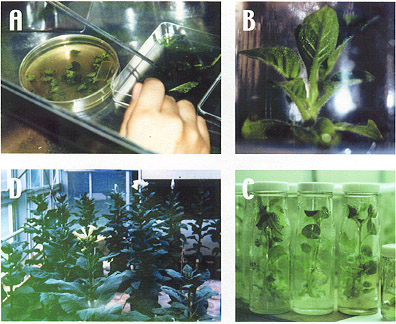
Fig. 4 Transformation
of chimeric PSPAL2 promoters into
tobacco plant.
A
: leaf disk transformation.
B :
young tobacco plant in the bottle.
C :
young tobacco plants before transferring in soil.
D
: transgenic tobacco plants in the greenhouse
|
 |
|
|
|
Fig. 5
GUS-PCR products from genomic DNA of the transgenic tobacco plants.
( GUS : b -
glucuronidase gene in pBI 101.2, M : l /HindIII, C : non-transformed plant
and No. 1-5 : transgenic plants ) |
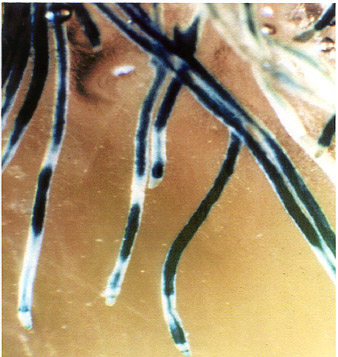 |
Fig. 6
Histochemical localization of GUS activity in young roots of transgenic tobacco plants
containing promoter of PSPAL2-FL-GUS-NOS construct. |
|
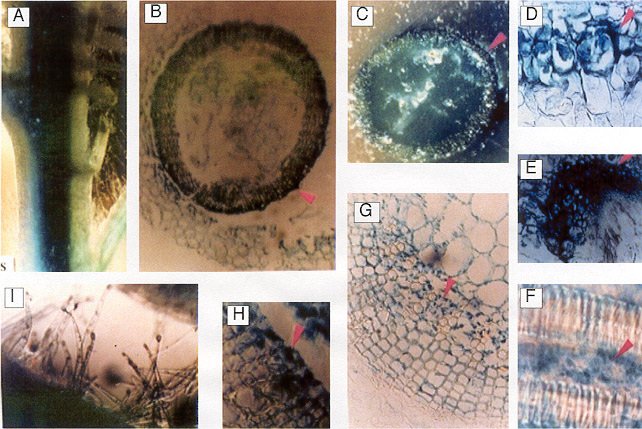
Fig. 7 Histochemical localization of GUS activity in young
stems both transverse and cross-sections of transgenic
tobacco plants containing promoter of PSPAL2-FL-GUS
-NOS construct.
A : vascular tissue of stem; B,C :
cross-section of stem
exhibiting high levels of GUS activity localized in the xylem rays (arrow) and in the
internal and external
phloem tissue;
D,E,G : closer view to the cross-section of stem; F,H
:
transverse-section of stem; I : stem trichomes
|
 |
|
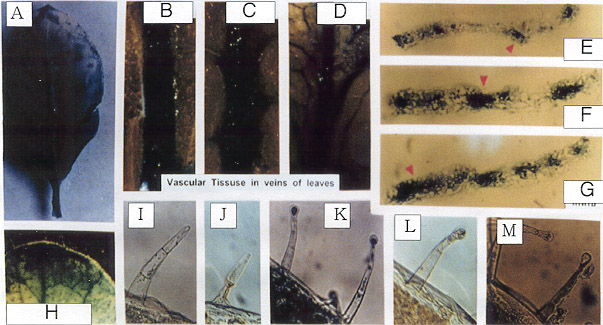 |
Fig. 8
Histochemical localization of GUS activity in young
leaves of transgenic tobacco plants containing promoter of
PSPAL2-FL-GUS-NOS construct.
A : whole
leaf; B-D : vascular in veins of leaves; E-G :
transverse-section in veins of leaves
H : leaf tip; I-M : leaf trichomes |
|
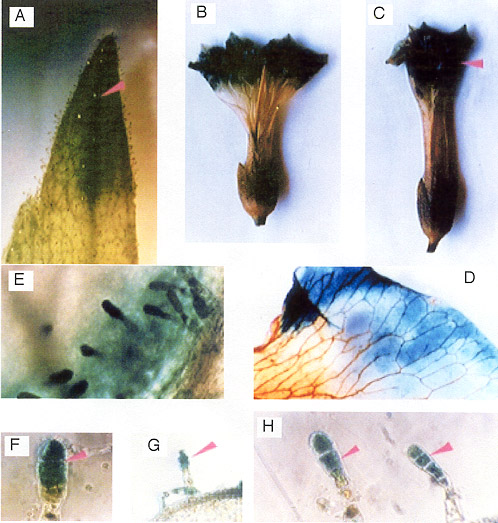
Fig. 9
Histochemical localization of GUS activity in flower
organ of transgenic tobacco plants containing promoter of
PSPAL2-FL-GUS-NOS construct.
A: sepal tip; B,C
:whole flower with high levels of GUS activity
localized in the petal; D: a portion of a petal, showing GUS
activity in the pigmented rim; E-H: gland cells of trichomes |
 |
|
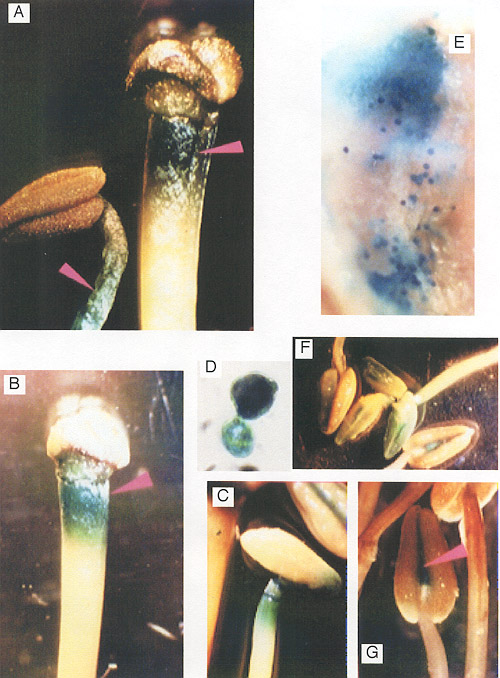 |
Fig. 10
Histochemical localization of GUS activity in of transgenic
tobacco plants containing promoter of PSPAL2-FL-GUS-NOS
promoter construct.
A-C :filaments; D-E
:pollens and anther wall ; F-G : anthers |
|
Fig. 11
GUS expression of the pea PSPAL2 full length
promoter (PSPAL2-FL, -2196 to +110) and three
deleted
chimeric promoters designated as PSPAL2-FLd1 (-1486
to +110), PSPAL2-FLd2 (-966 to +110) and PSPAL2-
FLd3 (-594 to +110) in leaves, stems and roots of
transgenic tobacco seedling during developmental
growth. |
 |
|
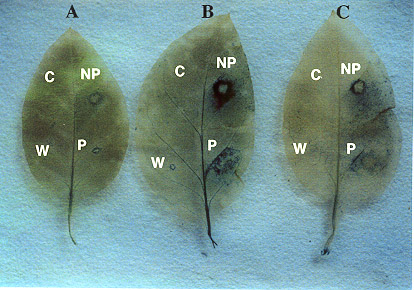 |
Fig. 12
Histochemical GUS expression of PSPAL2-FL promoter
upon wounding (W) and fungal ingression with a pathogen ( P, P.
nicotianae ) and non pathogen ( NP, P. capsici ) in transgenic
tobacco leaves. Control treatment is shown as C.
A: 24 hr after inoculation
or wounding
B: 48 hr after inoculation or wounding
C: 72 hr after inoculation or wounding
|
|
|
Fig. 13
Expression of full-length (PSPAL2-FL : A,E) and deleted PSPAL2
promoters (PSPAL2-FLd1 : B,F; PSPAL2-FLd2 :
C,G; PSPAL-FLd3 : D,H) at 48 hr after wounding (W) and
inoculation with a nonpathogen (NP, P. capsici) in transgenic
tobacco leaves. Bars equal 1 mm. |
|

