การยับยั้งกระบวนการแอนาม็อกด้วยอ็อกซีเตตร้าซัยคลิน Anammox Process Inhibition by Oxytetracycline |
|
INTRODUCTION To prevent harmful effects on human and aquatic life, nitrogen must be removed from wastewater before it is discharged into the environment. Conventional removal of nitrogen from wastewater by biological processes involves nitrification (NH4+ to NO2- to NO3-) followed by denitrification (NO3- to NO2- to N2). However, this process is difficult in high nitrogen but low carbon in wastewaters, such as effluents of anaerobic digestion or livestock and piggery waste after anaerobic treatment. The microbiological oxidation (anammox) process converts NO2- to N2 gas using NH4+ as electron donor, which saves energy, reduces carbon requirements, and decreases the biomass produced. However, antibiotics used in livestock and piggery farms could be discharged to wastewater. Oxytetracycline is commonly used in these farms. Furthermore, oxytetracycline is found in the produced manure from both livestock and piggery farms. For this reason, the goal of this study is to investigate the short term effect of oxytetracycline on the activity of anammox bacteria. METHODS Anammox culture was maintained in sequencing batch reactors (SBRs). Batch experiments were conducted using microorganisms harvested from the SBRs to examine the effect of oxytetracycline on anammox activity. Oxytetracycline experiments were done at initial nitrogen concentrations of 160-200 mg N/L, biomass concentrations of 300-500 mg VSS/L and oxytetracycline concentrations of 25, 50, and 100 mg/L. Monod-based inhibition models were used to describe oxytetracycline inhibition. Because the baseline rate of nitrogen removal is zero order with respect at nitrogen concentrations above 100 mg N/L, the following not competitive models were fit to the oxytetracycline inhibition of anammox data. The inhibition of nitrogen removal rate by oxytetracycline can be described by
where V is the reaction velocity (mg-N/L-hr), Vmax is the maximum reaction velocity (mg N/L-hr), I is the concentration of oxytetracycline inhibitor (mg/L), and Ki is the inhibition constant (mg/L). Reis et al. (1990) proposed a modified not competitive model that better captured the inhibition of sulfate-reducing bacteria. The model includes one additional parameter, a, as written below
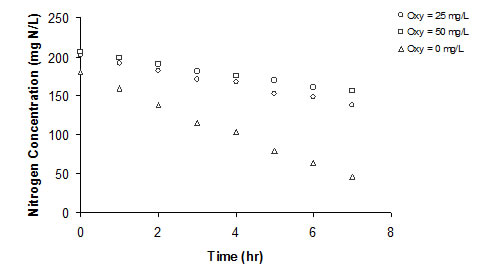
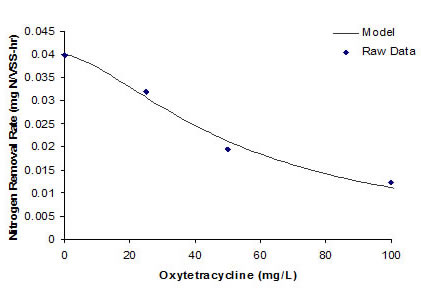
RESULTS Anaerobic ammonium oxidizer activity was inhibited by oxytetracycline concentration at 25, 50, and 100 mg/L. A control that contained no oxytetracycline (baseline rate), nitrogen removed 74.72%, nitrogen within seven hours. At oxytetracycline concentration of 25 and 50 mg/L, nitrogen removal efficiency was 33.52% and 29.37%, respectively (see Figure 1). Two forms of Monod-based inhibition models were used to describe oxytetracycline inhibition. Figure 2 shows the relative nitrogen removal rate as a function of oxytetracycline concentration and the Reis et al.’s model fits to the data well. Vmax is 0.03988 mg N/mg VSS-hr, Ki is 54.44 mg/L, and a is 1.55.
Figure 1 Nitrogen concentration vs. time at two oxytetracycline concentrations and biomass from 300 to 500 mg VSS/L
Figure 2 Nitrogen removal rate vs. oxytetracycline concentration. The line represents the best fit of the Reis et al model SUMMARY AND CONCLUSION Oxytetracycline is an inhibitor for anaerobic ammonium oxidizers affecting the activity of anammox culture in the short term. The Reis et al.’s model fits the data quite well, suggesting that oxytetracycline inhibition of anammox activity is a not-competitive phenomena. ACKNOWLEDGEMENTS The authors would like to thank grants from Faculty of Engineering, Kasetsart University, Bangkok, Thailand. Also, the authors would like to thank Department of Environmental Science, Faculty of Science, Silpakorn University, Nahkon-thom Province for significant support of this project. |
คณะผู้วิจัย
Pongsak (Lek) Noophan1*, Peerapas Narinkongnong2, Chalermraj Wantawin3, and Junko Munakata-Marr4
*1Department of Environmental Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, Thailand,*coresponding author (e-mail: fengpsn@ku.ac.th)
2Department of Environmental Science, Faculty of Science, Silpakorn University, Nakhon-pathom 73000, Thailand
3Department of Environmental Engineering, Faculty of Engineering, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand
4Environmental Science and Engineering Division, Colorado School of Mines, Golden, CO 80401, USA
โทร. 02-942-8555