The horse breeding in other countries have widely taken benefit from the use of cooled-stored semen. To study artificial insemination in native crossbred horse is useful method to increase this horse numbers and to improve equine breeding management in Thailand equally developing countries. This paper is report the first using artificial insemination technique with frozen semen in Thai native crossbred horse and a first foal from frozen semen.
The Thai native crossbred horse is generally used in religious ceremonies, especially in central part of Thailand such as Suphanburi, Ratchaburi, Kanchanaburi province. Moreover, Thai native crossbred horse is used for recreational activities and occasionally for transportation in highland areas such as Chiangrai, Chiangmai, Lampang province. Natural breeding with stallions is commonly performed to increase horse numbers in Thailand.
The use of artificial insemination (AI) in equine breeding has increased during recent decades and becoming important method in worldwide. AI offers many advantages over natural mating, such as safety for both mare and stallion, reduces risk of infectious disease transmission, and decrease inconvenience of horse transportation. Semen cryopreservation enhances the advantage of AI. Long-term storage facilitates semen transport over distances, permits the quarantine of semen, and enables extended use of semen, even after the sire’s death. Artificial insemination study in native crossbred horse is useful method to improve equine breeding management in Thailand equally developing countries.
Materials and method
1. Animals
Semen was collected from a Thai native crossbred stallion, aged 7 years old. The stallion was sexually rested for 1 week. A mare approximately 7 years old (judging the age by examining its teeth) was previously foaling in February 2007 and she had regular every 21 days of estrous cycle (figure 1).
2. Semen collection and cryopreservation
Semen was collected using a Missouri type artificial vagina while the stallion was mounting a teaser mare in oestrus in July 2007. Immediately after dismount the ejaculate was initially evaluated for total volume, gel-free volume, concentration, and motility. The original motility of sample needed to be least 50% to be considered acceptable for freezing in this project. Total number of spermatozoa was measured using a Neubauer counting chamber. After evaluation, semen was diluted at a ratio of 1:3 (semen: extender) in a Kenney extender (Kenney et al., 1975) and subjected to centrifuge at room temperature (30ºC) at 400 x g for 10 min in 15 ml capacity centrifuge tube to remove seminal plasma. Spermatozoa pellets were resuspended in Kenney freezing extenders with 3.5% glycerol to final concentration of approximately 200 x 106 sperm / ml and then placed in a passive cooling device (EquitainerTM) (5°C) for equilibration during transport to laboratory room for further processing. Semen sample was equilibrated at 5 °C for 2 h, and then loaded into 0.5 ml polyvinylchloride straws. Before freezing (BF), sample was evaluated motility, motion velocity, viability and membrane integrity. Prior to freezing, straws were sat in nitrogen vapor 3 cm above liquid nitrogen for 10 min, and then submerged in liquid nitrogen, finally stored in liquid nitrogen (-196°C).
3. Semen analysis
Frozen semen was thawed at 37°C water bath for 30 sec. Experimental endpoints included total sperm motility (TMOT; %), progressive motility (PMOT; %), and curvilinear velocity (VCL; µm/s), linear velocity (VSL, µm/s), average path velocity (VAP, µm/s), ALH, (BCF, Hz), (STR, %), and (LIN,%) as measured by computer assisted spermatozoal analysis (CASA; HTM–IVOS 12; Hamilton Thorne Research, Beverly, MA), by selecting five fields per sample. System parameters for CASA were 30 frames acquired at 60 frames per second; minimum contrast, 70; minimum cell size, 5 pixels; VAP cut-off, 10µm/s; and cut-off for progressive cells, 15 µm/s; VSL cut-off, 0 µm/s and straightness, 60%. The slow cells were considered static. A 3-µl drop of each sample was placed on a preheated (37ºC) 2X cell chamber (20 mm depth)
The functional plasma membrane integrity of frozen-thawed semen was performed with the hypoosmotic swelling test (HOS test; Neild et al., 1999), while live sperm was determined using the eosin-nigrosin staining tests (William, 2003).
4. Artificial insemination (AI)
4.1 Estrus detection
Mare was observed daily for visual signs of standing estrus. The ovaries were scanned by ultrasonography twice daily for at least 35 mm diameter of a preovulatory follicle detection.
4.2 Artificial Insemination technique
According to previously cycle record, ovulation was occurred from follicle with around 40 mm diameter. Thus, AI was performed once when the follicle was approximately 40 mm diameter and the uterus with slightly edematous endometrial gland was observed. The mare was secured in breeding stocks, the tail was wrapped and deflected to one side. One 0.5 ml straw of semen containing approximately 100 x 106 spermatozoa was thawed at 37 °C for 30 seconds, and then diluted into 30 ml of Kenney extender. Semen was inseminated using a sterile rigid insemination tube toward the uterine horn, in which ovulation was presumed to occur on 28th July, 2008. Pregnancy diagnosis was performed at 18, 25 and 35 days after ovulation using ultrasonography
Result
The color of collected semen was milky white. Gel free-volume, total motility (TMOT), progressive motility (PMOT), viability and concentration of fresh semen were 35 ml, 90%, 80%, 94% and 135 x106 sperm/ml respectively. The TMOT, PMOT, lived sperm and HOST positive membrane integrity of sperm before freezing (BF) and post-thawed (PT) were shown in Table 1.
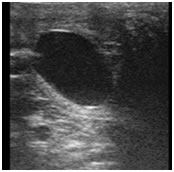
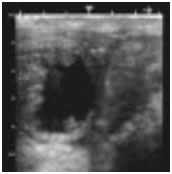
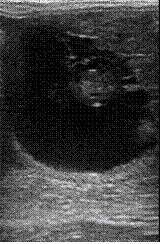
. A dominant follicle was observed in the right ovary. Mare was inseminated once and ovulation was occurred within 6 hours after AI. A single embryo sac with embryo was detected in one uterine horn at 18th, 25th and 35th day after ovulation (figure 2).
Table 1 Motility, motility pattern, lived and HOS test positive sperm before freezing (BF) and post-thawed (PT) stallion semen
Parameter |
BF |
PT |
TMOT (%) |
90 |
48 |
PMOT (%) |
85 |
32 |
VAP (µm/s) |
129.1 |
88.6 |
VSL(µm/s) |
81.5 |
72.9 |
VCL(µm/s) |
240.6 |
164.0 |
ALH |
9.4 |
7.2 |
BCF (Hz) |
38.7 |
41.5 |
STR (%) |
60 |
76 |
LIN (%) |
33 |
41 |
Area (µm2) |
5.5 |
5.4 |
Rapid cell (%) |
86 |
40 |
Static cell (%) |
1 |
51 |
Lived sperm (%) |
94 |
47 |
HOST positive sperm (%) |
78 |
40 |
The pregnant mare was foaling a colt on 17th June, 2009. The gestation was 319 days long. A newborn colt is healthy, standing within 10 minutes. A newborn colt has 37 kg., and 83 cm. height and has white-brown color (figure 1).
 |
 |
a |
b |
|
|
c |
d |
Figure1 Thai crossbred stallion (a), Thai native mare (b), and the foaling (c,d) performance.
Figure 2 Thai native crossbred embryo after insemination with frozen semen a) at 18 days; b) at 25 days; and c) at 35 days old.
Discussion
Semen extenders are mainly based on milk, with a composition similar to original recipe published by Kenney et al. (1975) are most popular and used worldwide. This extender is inexpensive, easy to prepare, can be stored in frozen form and result in acceptable fertility rate.
Insemination with frozen semen, timing the ovulation is highly desirable in order to reduce the interval between breeding and ovulation. This insemination time was examined by transrectal palpation and ultrasonography every 8 h when the mare is displaying behavioral estrus and has a follicle greater than 35 mm. to increase of accuracy in the timing of breeding. However, using ovulation inducing agents such as human chorionic gonadotropin (hCG) or the GnRH analogue, deslorelin, are critical components to accurately time the insemination with frozen semen (Samper, 2001).
The gestational length in this AI mare is shorter than average length, however the gestational length of pony mare is shorter (Michelle et al., 2000).
Foal birth weights are perceived to be important within the commercial Thoroughbred industry of Australia, as foal birth weight is commonly thought to positively associate with size as yearling. There is a significant positive correlation between birth height and mature height, concluded that birth height could be used as an accurate predictor of mature height (Reed and Dunn, 1977). However, birth weight and birth height measurement, and the relationship between these two measurements have not been investigated in horses in Thailand. The birth weight of this AI colt is lower than Thoroughbred colts, might be cause of different breed and nutrition.
Conclusion
In conclusion, the results of the present study demonstrated that Thai native crossbred stallion sperm could be frozen and resulted in the birth of normal foal after artificial insemination to the native crossbred mare. This is the report of the first foal using frozen semen for artificial insemination in equine species in Thailand.
References
- Kenney, R.M., R.V. Bergman, W.L. Cooper and G.W. Morse. 1975. Minimal contamination techniques for breeding mares. Proc Am Ass Eq Pract. 21: 327-336.
- Michelle, M.L., R.J. Rose, and D.R. Hodgson. Reproduction. 2000. In R.J. Rose, and D.R. Hodgson. Manual of equine practice. 2nd eds. W.B. Saunders company. Philadelphia, USA. p 341-380.
- Neild, D., G. Chaves, M. Flores, N. Mora, M. Beconi and A. Aguero. 1999. Hypoosmotic test in equine spermatozoa. Theriogenology. 51: 721-727.
- Reed, R., and N. Dunn. 1977. Growth and development of the Arabian horse. In: Proceedings of the fifth equine nutrition physiology symposium. p. 76-98.
- Samper, J.C. 2001. Management and fertility of mares bred with frozen semen. Anim Reprod Sci. 68:219-228.
- William, M.B. 2003. Predictive value of hypo-osmotic swelling test to identify viable non-motile sperm. Asian J Androl. 5: 209-212.
|