เพื่อประยุกต์ใช้เป็นแผ่นเยื่อบางอิเล็กโทรไลต์ของเซลล์เชื้อเพลิงของแข็ง
Preparation of Cerium Ceramic Powder from Metal-Organic Precursor via One Pot Process
for Application as Solid Oxide Fuel Cell Electrolyte Membrane
1Department of Chemical Science and Technology, University of Rome "Tor Vergata", Roma, Italy
2Department of Chemistry, Faculty of Science, Kasetsart University, Bangkok, Thailand
3Department of Materials Science and Engineering, Faculty of Engineering and Industrial Technology,
Silpakorn University, Nakorn Pathom, Thailand
4Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok, Thailand
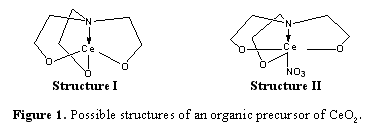
| CeO2 precursor was successfully prepared via the One Pot process. On the basis of mass spectroscopy, the precursor was proposed to be monometallic species consisting of one TEA group per metal ion or a monometallic species consisting of one TEA group per metal ion and a nitrate group. The precursor was converted to CeO2 by sintering at 600, 800, and 1000oC for 2 h in air. Light yellowish colored powders were studied by XRD. Introduction : Fuel cells are expected for a practical power generation and environmental friendly devices converting the chemical energy directly into electrical energy under the processes of electrochemical reaction. Solid oxide fuel cells, based on oxide-ion conducting electrolyte, offer clean, low-pollution technology to electrochemically generate electricity at high efficiencies. Up to now, various researches have been focused on the improvement of the SOFCs performance and stability. Electrolytes providing high oxide ion conductivity and being operated at low temperature are developed. Ceria (CeO2) is a fluorite-structured oxide that can form extensive solid solutions with a variety of cations while keeping the fluorite crystal structure. Doped ceria has been considered as one of the most promising electrolyte materials for intermediate temperature-solid oxide fuel cells. Chemical routes to ceramics offer many advantages over traditional methods, including the potential to control product homogeneity and purity, to lower processing temperatures. One of them called the one pot process, is very simple and straightforward. It provides high purity and homogeneity product. Due to the advantage as mentioned above, in this research work, metal-organic precursor of cerium is prepared via one pot synthesis. Methodology: Ce(NO3)3.6H2O and triethanolamine were reacted in n-propanol for 4 h. The solid products were characterized by ESI-MS, TGA, and FTIR. The precursor was sintered in a furnace for 2 h with various temperatures such as 600, 800 and 1000oC. The powder was characterized by XRD. Results and Discussion: The reactions of Ce(NO3)3.6H2O and TEA were proceeded in n-proponol to obtain the homogeneous solution. The yellowish powder was precipitated when the reactants were completed reacted for 4 h. The FTIR spectrum of precursor showed C-H stretching bands occurred in the region 2928 and 2890 cm-1 was assigned for -CH2- group in the product. In addition, the resonance at 1080 cm-1 was ascribed to the Ce-O-C stretching vibration, and the band at 550 cm-1 is due to the Ce-O stretching. The precursor structure was then characterized by using electrospray ionization (ESI) Mass spectroscopy. Two significant peaks were generated at m/z = 286 and 348, respectively. However, these two peaks exhibited in different intensity. The possible structures of product (Figure 1) were proposed to be a monometallic species consisting of one TEA group per metal ion (Structure I) and a monometallic species consisting of one TEA group per metal ion and a nitrate group (Structure II). The appropriate temperature for sintering was studied by TGA. Two weight loss regions appeared in the TGA thermogram. It was found that after 800oC, no weight loss was generated; the appropriate sintered temperatures were therefore varied in the range of 800-1000oC for preparing CeO2 powder. In addition, the ceramic yield was found to be 32%. The precursors were pyrolyzed at various temperatures, such as 600, 800, and 1000oC for 2 h in air. Light yellowish colored powders were characterized by powder X-ray diffraction (XRD). Peak positions of all products were identified corresponded to the fluorite structure by comparing with JCPDS file No. 34-0394. The XRD patterns of all products were not different in positions as comparing with the standard XRD pattern
Conclusion: The One Pot Process was successfully used to prepare the CeO2 powder. This method also offers an easy, inexpensive, straightforward alternative to solid-solid reaction, co-precipitation and other chemical techniques of ceramics processing, and retains the advantages of purity, homogeneity, and low processing temperatures. The powders will be further studied in the second stage as following the research plan concerned with application as an electrolyte membrane for solid oxide fuel cell. |